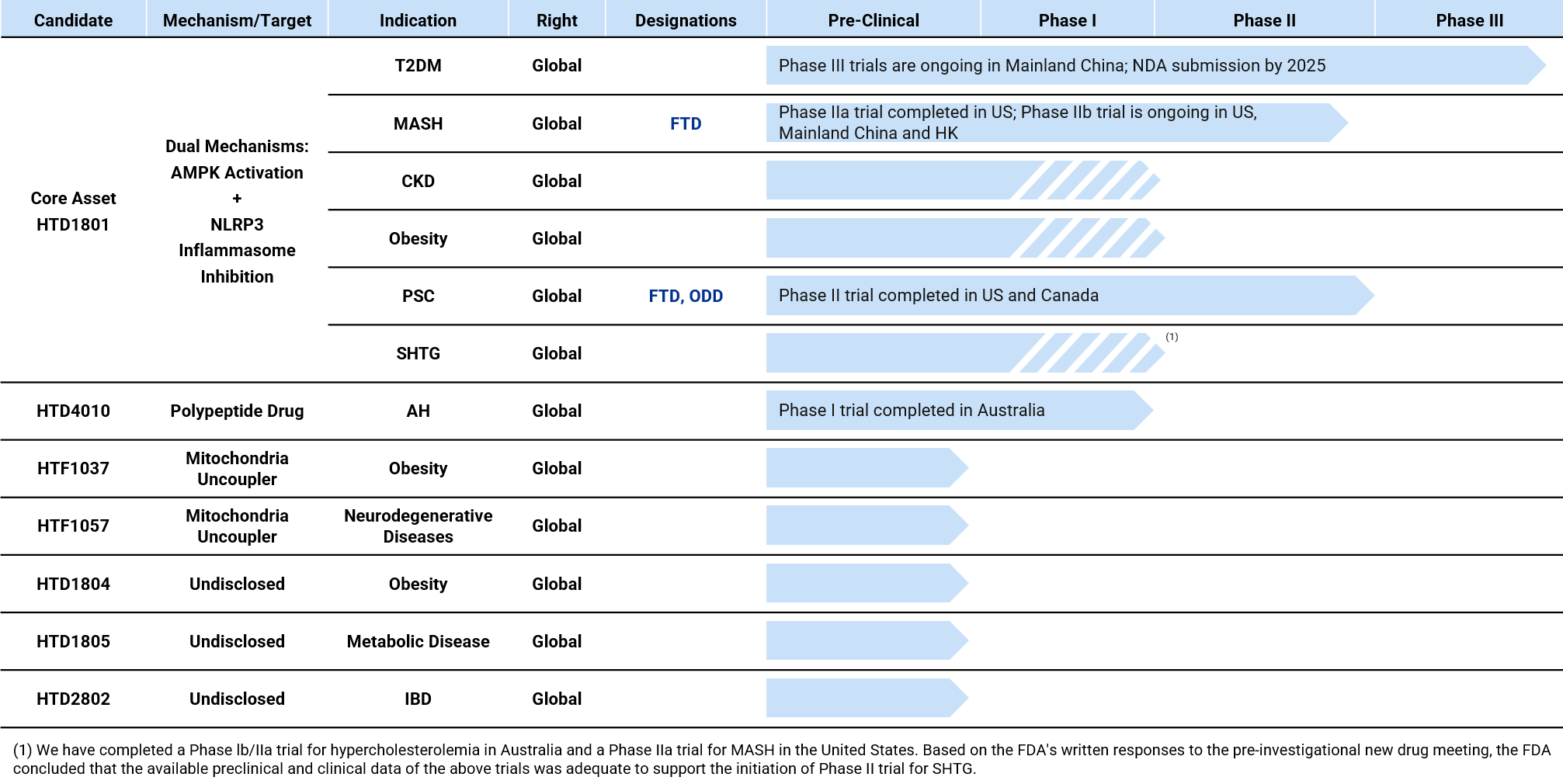
HighTide Therapeutics develops its pipeline candidates in accordance with international standards and has conducted multiple global clinical trials in China, the United States, Australia, and Canada, with safety and efficacy validated across multiple studies.
Updated August 2025