June 24, 2023
ROCKVILLE, MD and SHENZHEN, CHINA, June 24, 2023— HighTide Therapeutics, Inc. (“HighTide”) announces that data from the Phase 2a clinical trial of HTD1801, a first-in-class gut-liver anti-inflammatory metabolic modulator for patients with nonalcoholic steatohepatitis (NASH) and type 2 diabetes mellitus (T2DM), will be presented at the European Association for the Study of the Liver (EASL) Congress 2023, to be held June 21-24, 2023 in Vienna, Austria. The presentation features results of a post-hoc analysis exploring the effect of HTD1801 treatment on corrected T1 (cT1), an MRI-based quantitative metric for assessing liver inflammation and fibrosis.
This trial was an 18-week randomized, double-blind, placebo-controlled Phase 2a study that was designed to evaluate the efficacy and safety of HTD1801 compared to placebo in adult patients with NASH and T2DM. One hundred patients with NASH and T2DM were randomized into 3 treatment groups: one of two doses of HTD1801 (500 mg BID and 1000 mg BID) or placebo. The study met the primary endpoint (liver fat content [LFC] by MRI proton density fat fraction [MRI-PDFF]) and achieved clinically important secondary endpoints related to improvements in liver health and metabolism. HTD1801 was generally safe and well tolerated. This post-hoc analysis, focused on cT1, to be presented at the EASL Congress 2023 provides further evidence that HTD1801 may improve histologic measures of disease activity in patients with NASH and T2DM.
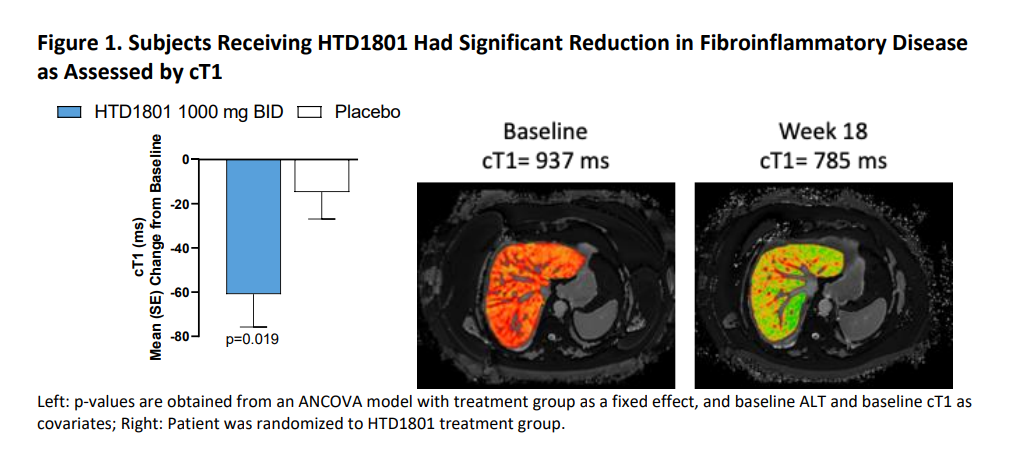
cT1 has been shown to be elevated in patients with NASH as a measure of fibroinflammation in the liver with reductions in cT1 significantly associated with histologic improvements. In this study, cT1 levels were elevated at baseline. After 18 weeks of treatment, there was a significant reduction in cT1 with HTD1801 1000mg BID compared to placebo (-60.9 [75.9] ms vs -14.7 [68.9] ms, p<0.05). Furthermore, a larger proportion of subjects receiving HTD1801 1000mg BID compared to placebo (39% vs 16%, respectively) experienced at least an 80 ms reduction in cT1, which has been correlated with a 2-point reduction in the NAFLD activity score (NAS).
“Across multiple biomarkers, HTD1801 resulted in more patients achieving clinically relevant thresholds correlated with histologic improvement and lower disease activity. These data suggest that HTD1801 may improve liver histology in patients with NASH and T2DM, and merits further development as a treatment for NASH with T2DM,” said Stephen Harrison, M.D., a leading expert in NASH and the principal investigator for the study.
"We are greatly encouraged by the observed reduction in fibroinflammatory disease based on this post-hoc analysis, which continues to show the therapeutic potential of HTD1801 in patients with NASH and T2DM. Based on this latest data set, we continue to believe HTD1801 has the potential to play an important role in treating patients with NASH. A Phase 2b study is currently ongoing to evaluate the histologic effects of HTD1801 in patients with NASH and confirm the findings of this evaluation," said Dr. Liping Liu, Founder, and CEO of HighTide.
About NASH
NASH is a severe progressive liver disease caused by excessive fat accumulation in the liver that induces chronic inflammation, resulting in progressive fibrosis that can lead to cirrhosis, liver failure, cancer, and death. Patients with NASH and T2DM or impaired glucose tolerance are more likely to progress to more severe disease and to develop complications that lead to increased mortality. Prevalence of NASH is on the rise and is the fastest growing cause of liver transplants and liver cancer in the US and Europe. Currently, there are no approved therapies for NASH.
About HighTide
HighTide Therapeutics, Inc. is a globally integrated biopharmaceutical company focusing on the discovery and development of first-in-class, multifunctional, multi-targeted therapies for the treatment of metabolic and digestive diseases with significant unmet medical needs. The company is developing multiple clinical assets, including therapy for NASH, T2DM, severe hypertriglyceridemia (SHTG), primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC). HTD1801, the company’s lead drug candidate, received Fast Track designation from the U.S. for both NASH and PSC, as well as Orphan Drug designation for PSC. In China, HTD1801 has been included in the National Major New Drug Innovation Program under the 13th Five-Year Plan for Major Technology Project.
For more information, please visit www.hightidetx.com, Contact: pr@hightidetx.com.

We will contact you as soon as possible
© 2024 HighTide Therapeutics, Inc.